
Export of Measuring Instruments with EU CE Certification
In 2014, the EU issued the new Measuring Instruments Directive (MID) 2014/32/EU, which started to be implemented in April 2016, replacing the previous directive 2004/22/EC. MID is the EU regULation for overseeing and managing measuring instruments, with the aim of establishing a single market for measuring instruments for manufacturers. Once a manufacturer obtains a certificate, their products can freely circulate across the entire EU market.
Certification Scope of the MID Directive
The MID directive provides the basic requirements and conformity assessment procedures for all measuring instruments, and special requirements are provided for instruments within ten specific categories:
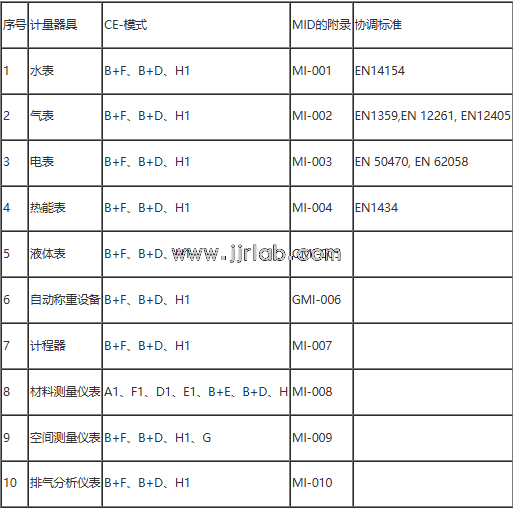
Note: The MID directive does not mention non-automatic weighing instruments. These non-automatic weighing instruments are still governed by the NAWI directive, which has not changed. For those without specific harmonized standards, CE certification is carried out according to the directive's standards.
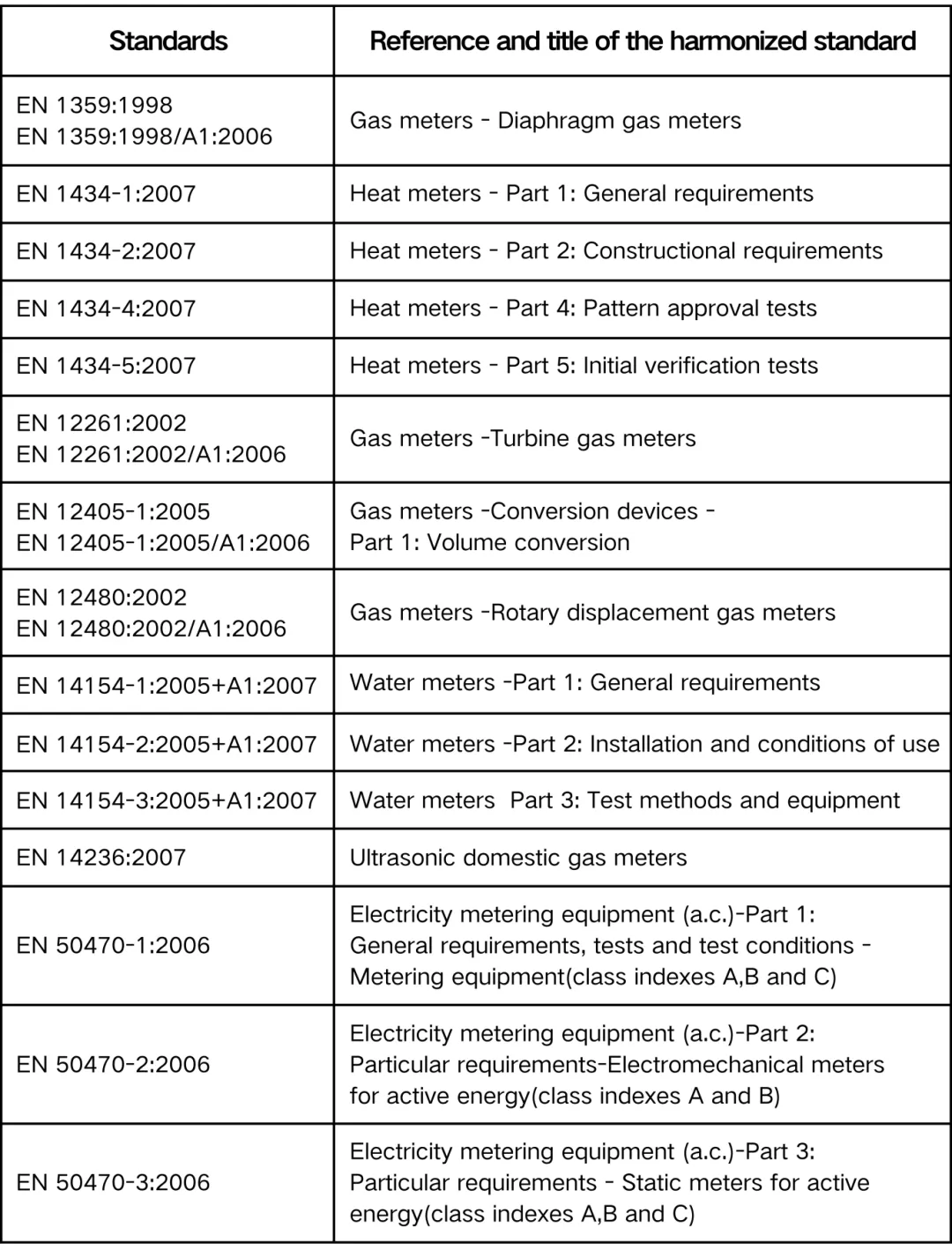
Main Harmonized Standards
Certification Modes of the MID Directive
For different types of measuring instruments, the MID provides conformity assessment procedures in the relevant annexes. For example, for gas meters, MI-002 specifies that producers can choose between B + F, B + D, or H1 conformity assessment procedures.
- Mode A: Based on internal production control and Declaration of Conformity (DoC) (A1 = A + product testing by a notified body);
- Mode B: Type examination by a notified body;
- Mode C: Declaration of Conformity (DoC) based on internal production control (C1 = C + product testing by a notified body);
- Mode D: Type DoC based on Quality Assurance (QA) of the production process (D1 = DoC based on production process);
- Mode E: Type DoC based on QA of final product inspection and testing (E1 = DoC based on QA of final product inspection and testing);
- Mode F: Type DoC based on product certification by a notified body (F1 = DoC based on product certification by a notified body);
- Mode G: Type DoC based on product certification of individual units by a notified body;
- Mode H: Type DoC based on a comprehensive quality assurance system (A1 = product testing by a notified body).
Terminology Definitions:
DoC: Declaration of Conformity, QA: Quality Assurance
All modes are detailed in the MID directive. The letters A, B, … H represent the certification mode identifiers, while the number 1 (e.g., A1, C1, … H1) indicates minor variations in additional characters. They are essentially consistent in principle, with only small differences.
Technical Documentation Requirements of the MID Directive
1. Technical documentation should clearly present the design, manufacture, and operation of the measuring instrument, allowing for the assessment of its compliance with the applicable requirements of this directive.
2. Technical documentation should be sufficiently detailed to ensure compliance with the 2014/32/EU directive.
3. The technical documentation should include the necessary information to assess and identify the scope related to the type/measuring instrument.
4. The manufacturer should specify where seals and markings have been applied.
5. The manufacturer should indicate, where relevant, the conditions of compatibility with interfaces and subcomponents.
Specific requirements can be consulted with OUCE (EU Measurement) for further details.
Email:hello@jjrlab.com
Write your message here and send it to us
 Cost of U.S. FDA CFR 21 177.2600 Test Report
Cost of U.S. FDA CFR 21 177.2600 Test Report
 How much does the IP44 Compliance Test cost
How much does the IP44 Compliance Test cost
 What is LFGB Test
What is LFGB Test
 What does LFGB certified mean?
What does LFGB certified mean?
 Weee authorised representative germany
Weee authorised representative germany
 Where to Apply for 2026 Air & Sea Transport Ce
Where to Apply for 2026 Air & Sea Transport Ce
 Guide to IEC Test Reports for Lighting Exports
Guide to IEC Test Reports for Lighting Exports
 IEC/EN 62471 and IEC/EN 62778 (Photobiological Saf
IEC/EN 62471 and IEC/EN 62778 (Photobiological Saf
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




