
FDA IEC TR 60601-4-2 Standard Testing
In the field of medical devices, the International Electrotechnical Commission (IEC) has developed two critical standards to ensure electromagnetic compatibility (EMC): iec 60601-4-2 and IEC 60601-1-2. While these two standards share certain similarities, they focus on different aspects of emc compliance.

Currently, only the FDA has officially released a new EMC guidance document to replace the 2016 version. Neither the EU nor China has plans to develop an EN-equivalent standard of IEC TR 60601-4-2. Today, our focus is to understand the IEC TR 60601-4-2 standard.
Understanding IEC TR 60601-4-2
Recently, during 510(k) project discussions, many clients have asked why the IEC test submission list includes a report for IEC 60601-4-2. To address this, we will explore the similarities and differences between the two standards.
IEC TR 60601-4-2 is a technical report specifically focused on EMC immunity. Unlike IEC 60601-1-2, which concentrates on basic safety and essential performance, IEC 60601-4-2 emphasizes the performance criteria under electromagnetic immunity tests based on the intended use of medical electrical equipment.

For example, a device may be affected by EMC but still remain safe; however, a device that claims to be safe might not be effective. IEC TR 60601-4-2 addresses performance criteria beyond basic pass/fail definitions of essential performance.
Published in 2016, this technical report complements IEC 60601-1-2 by guiding manufacturers and testing bodies on how to consider and define performance criteria and immunity test levels. However, since it's a supplementary document and not mandatory in most regions, it is often overlooked.
Structure of IEC TR 60601-4-2
By examining the table of contents, we can see that the structure is similar to IEC 60601-1, provided that performance criteria are clearly defined. The standard also clarifies that if performance under IEC 60601-1-2 immunity levels is met, further testing may not be necessary.
Depending on the intended use and environment, th

e guideline recommends different considerations for EMC immunity. Annex C of IEC 60601-4-2 provides detailed examples, which we won’t expand on here.

As stated in the preface, the FDA requires evidence of compliance with IEC TR 60601-4-2 because the newly published EMC guidance refers to the recommendations in this document mULtiple times, as shown below:
> — It is recommended to include corresponding descriptions to demonstrate that the product meets its intended performance.
Example from Annexes of IEC TR 60601-4-2
IEC 60601-4-2:2016 Annex D provides examples related to functions and performance indicators of ultrasound and imaging systems, emphasizing that the observed variations and deviations do not affect final diagnosis or treatment.
Similarly, for monitoring devices such as pulse oximeters and blood pressure monitors, the performance criteria can be defined according to clinical scenarios and risk analysis, considering the device design.
In addition to performance criteria for individual functions, IEC 60601-4-2 also includes examples for overall system immunity:

> IEC 60601-4-2:2016 Annex D
For system-level equipment, the description of immunity criteria is no longer limited to single functions. Instead, it includes multiple stages such as startup, positioning, and final display, each with its corresponding criteria.
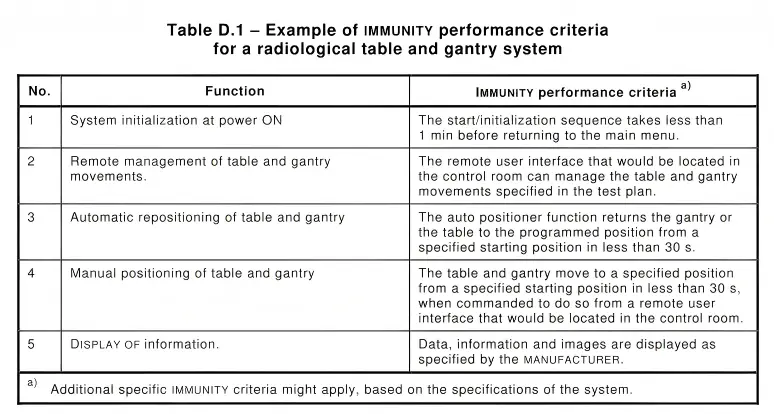
These immunity criteria are crucial for testing laboratories during the evaluation process. Clear and detailed descriptions help laboratories quickly identify and resolve issues during testing, REDucing repeated communication and accelerating project progress.
Summary
IEC 60601-1-2 defines the EMC requirements related to medical device safety, but does not cover the performance of the device in various electromagnetic environments. In contrast, IEC 60601-4-2 addresses comprehensive performance issues under electromagnetic interference. While the two documents are related, their acceptance criteria differ significantly.
Compliance with IEC TR 60601-4-2 may be more challenging than compliance with IEC 60601-1-2.
Therefore, when working on 510(k) projects, there is no need to debate which standard applies — evidence of compliance with both IEC 60601-1-2 and IEC TR 60601-4-2 must be submitted to meet EMC requirements.
More: uk psti | 5g compliance testing | emi testing labs | weee registration
Email:hello@jjrlab.com
Write your message here and send it to us
 European Toy Safety Standard EN 71-20:2025
European Toy Safety Standard EN 71-20:2025
 EN 18031 Certification for Connected Devices on Am
EN 18031 Certification for Connected Devices on Am
 Compliance Guide for Portable Batteries on Amazon
Compliance Guide for Portable Batteries on Amazon
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
 LFGB Certification Cost and Timeline Guide
LFGB Certification Cost and Timeline Guide
 Bluetooth FCC Test Report
Bluetooth FCC Test Report
 Is FCC Testing Required?
Is FCC Testing Required?
 Where to Find FCC Test Reports
Where to Find FCC Test Reports
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




