
IVD Medical Device GB 4793:2024 Test Report
With the rapid advancement of medical technology, China’s regULatory system is also continuously improving. Since 2023, the GB 4793 series standards concerning domestic IVD (In Vitro Diagnostic) devices have undergone updates. The government has issued the mandatory GB 4793—2024 standard along with several recommended GB/T 42125 series standards. To keep pace with industry trends and ensure product compliance and market competitiveness, enterprises must stay informed and prepare early for these new standards.

The GB 4793—2024 mandatory national standard was released on October 28, 2024, and will be officially implemented starting November 1, 2026. It applies to IVD medical devices as well as electrical equipment used in measurement, control, and laboratory environments. The standard outlines safety requirements during product design, production, testing, and usage. Key technical requirements and their corresponding test methods are as follows:
1. Markings and Documentation – Tested according to Chapter 5 of GB/T 42125.1-2024
2. Protection Against Electric Shock – Tested according to Chapter 6 of GB/T 42125.1-2024
3. Protection Against Mechanical Hazards – Tested according to Chapter 7 of GB/T 42125.1-2024
4. Resistance to Mechanical Stress – Tested according to Chapter 8 of GB/T 42125.1-2024
5. Flame Spread Prevention – Tested according to Chapter 9 of GB/T 42125.1-2024
6. Temperature Limits and Heat Resistance – Tested according to Chapter 10 of GB/T 42125.1-2024
7. Protection Against Liquid and Solid Ingress – Tested according to Chapter 11 of GB/T 42125.1-2024
8. Protection from Radiation (including lasers), Sound, and Ultrasound Pressure – Tested according to Chapter 12 of GB/T 42125.1-2024
9. Protection Against Released Gases, Explosions, and Implosions – Tested according to Chapter 13 of GB/T 42125.1-2024
10. Component and Assembly Safety – Tested according to Chapter 14 of GB/T 42125.1-2024
11. Protection via Interlocking Devices – Tested according to Chapter 15 of GB/T 42125.1-2024
To support the implementation of GB 4793—2024, several GB/T 42125 series standards have been issued. These new standards gradually replace the older GB 4793 series and introduce updated specifications related to dielectric strength, optical radiation, sound pressure levels, application risks, and risk assessment. Notably, YY 0648-2008, the standard for IVD medical device safety, has not yet been updated.
New vs Old Standards Overview:
- GB 4793-2024 replaces GB 4793.1-2007, released on 2024-10-28, effective from 2026-11-01
- GB/T 42125.1-2024 replaces GB 4793.1-2007, released on 2024-10-26, effective from 2026-11-01
- GB/T 42125.2-2024 replaces GB 4793.6-2008, released on 2024-11-28, effective from 2026-12-01
- GB/T 42125.5-2024 replaces GB 4793.7-2008, released on 2024-11-28, effective from 2026-12-01
- GB/T 42125.14-2023 replaces GB 4793.9-2013, released on 2023-03-17, effective from 2024-04-01
About JJR Laboratory
JJR Laboratory in China spans over 30,000 square meters, including more than 10,000 square meters of testing areas. It is equipped with 13 specialized testing platforms covering:
- Electromagnetic compatibility
- Electrical safety
- Wireless communication
- Biosafety
- MICrobiology
- Cleaning and disinfection validation
- Specific product performance
- Mechanical and physical tests
- Chemical analysis
- Environmental reliability
- Optical and acoustic measurements
- Software testing
The lab is recognized or supported by national and local medical product authorities. For over two decades, it has operated under ISO/IEC 17025 standards. JJR holds CMA certification and is accREDited by CNAS and IAS. It is also a recognized CBTL, and OECD GLP laboratory, with authorizations from fcc, IC, and VCCI. The lab has approved facilities for small and large laboratory animals, enabling testing on monkeys, pigs, dogs, sheep, rabbits, and rodents. Its test reports are internationally credible.
Email:hello@jjrlab.com
Write your message here and send it to us
 What is Protection Class EN 60529?
What is Protection Class EN 60529?
 IP69 Certified Protection
IP69 Certified Protection
 California Energy Commission Testing Lab
California Energy Commission Testing Lab
 What Does the Canadian IC Mark Mean?
What Does the Canadian IC Mark Mean?
 How Much is the Canada IC ID Certification cost?
How Much is the Canada IC ID Certification cost?
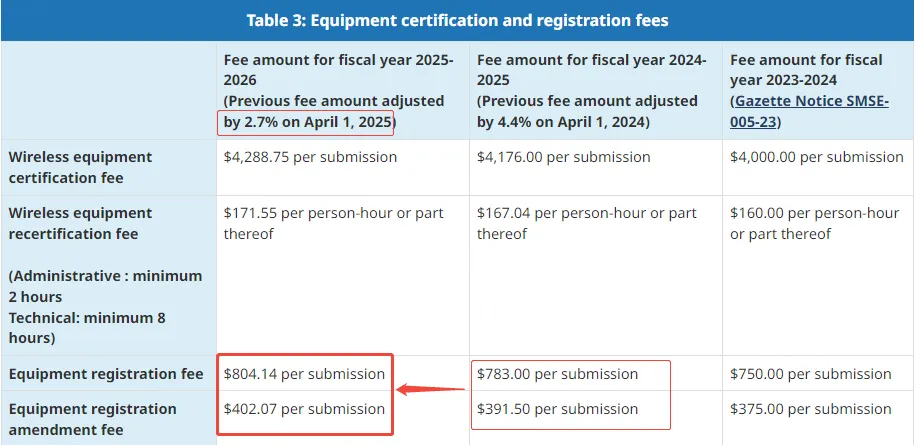 How Much is the Canada IC ID Certification Fee?
How Much is the Canada IC ID Certification Fee?
 How Much is the UL 982 Test Report csost?
How Much is the UL 982 Test Report csost?
 ISO/IEC 17025 Accredited Test Laboratory
ISO/IEC 17025 Accredited Test Laboratory
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




