
What is a Dun & Bradstreet (D-U-N-S®) Number?
When medical device companies undergo FDA registration certification, they often encounter a term - "Dun & Bradstreet (D-U-N-S®) Number." Applying for a Dun & Bradstreet (D-U-N-S®) Number is a necessary prerequisite for companies to successfULly complete FDA registration certification because it is requiRED for establishing a company's paid account and applying for a UDI database account, among other steps. Today, JJRLAB is here to provide a detailed introduction to the Dun & Bradstreet (D-U-N-S®) Number.

What is a Dun & Bradstreet (D-U-N-S®) Number?
The Dun & Bradstreet (D-U-N-S®) Number is a real-time dynaMIC enterprise identification consisting of 9 digits. It originates from the global coding system developed and managed by Dun & Bradstreet. It is widely used for enterprise identification, organization, and arrangement of business information, and can be used to identify and locate enterprise information quickly for risk management purposes.
Why does the FDA require a Dun & Bradstreet (D-U-N-S®) Number?
As a globally recognized enterprise identification code, the Dun & Bradstreet (D-U-N-S®) Number is unique, with each enterprise entity having a corresponding code that is not reused, similar to an enterprise's ID number.
- When applying for a Dun & Bradstreet (D-U-N-S®) Number, companies need to submit information such as name and address. The FDA can identify and verify companies through the Dun & Bradstreet (D-U-N-S®) Number, which helps improve the quality and accuracy of FDA registration data.
- The Dun & Bradstreet (D-U-N-S®) Number is closely related to the credit evaluation of companies. The FDA can quickly and accurately check a company's history records, credit records, etc., through the Dun & Bradstreet (D-U-N-S®) Number to ensure the quality and safety of products.
Dun & Bradstreet (D-U-N-S®) Number Application Checklist
- Company name in Chinese and English
- Company office address in Chinese and English
- Mailing address (if different from the office address, this information needs to be submitted)
- Name and position of company manager
- Number of employees
- Business contact person
- Phone number
- Position
- Type of business
- Scanned copy of business license
- Company authorization letter with seal and signature
JJR Advantage
JJRLAB is a "one-stop" medical device product quality service platform that integrates medical device product testing, certification, technical regulatory consultation, and clinical verification. With over twenty years of experience and technological innovation, JJRLAB has a technical team with both reputation and strength, capable of assisting medical device companies in quickly and accurately applying for a Dun & Bradstreet (D-U-N-S®) Number and providing them with one-stop medical device FDA registration certification services, helping companies smoothly enter the U.S. market.
Email:hello@jjrlab.com
Write your message here and send it to us
 JJRLAB's New Chemical Laboratory Expansion Complet
JJRLAB's New Chemical Laboratory Expansion Complet
 JJRLAB 5G NR and Sub 6G communication testing
JJRLAB 5G NR and Sub 6G communication testing
 JJRLAB Completes Expansion of CPSC Full Project
JJRLAB Completes Expansion of CPSC Full Project
 What certifications for Middle East exports?
What certifications for Middle East exports?
 US-bound button cell batteries need UL4200A-2023
US-bound button cell batteries need UL4200A-2023
 Button batteries ANSI/UL 4200A-2023 Laboratory
Button batteries ANSI/UL 4200A-2023 Laboratory
 What is a Dun & Bradstreet (D-U-N-S®) Number?
What is a Dun & Bradstreet (D-U-N-S®) Number?
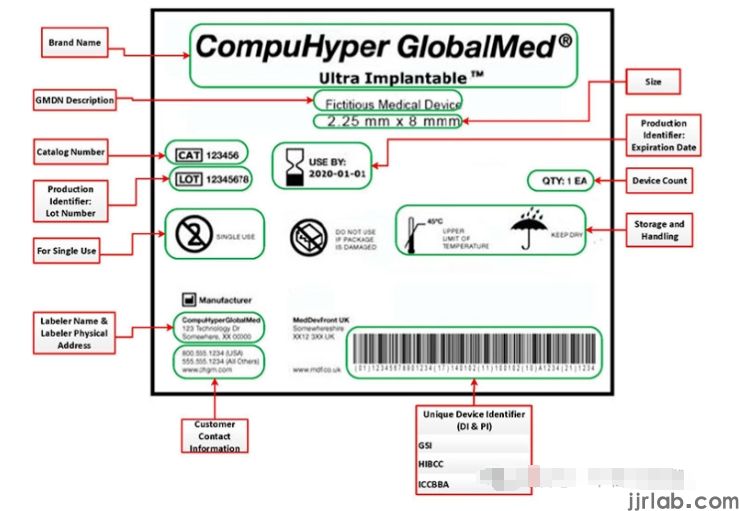 The Compliance Process of UDI with the U.S. FDA
The Compliance Process of UDI with the U.S. FDA
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




