
GB/T 18268 Series Standard Requirement
GB/T 18268.1-2010 is the national standard "Electromagnetic Compatibility Requirements for Electrical Equipment for Measurement, Control, and Laboratory Use - Part 1: General Requirements". This standard stipULates the electromagnetic compatibility (EMC) requirements for electrical equipment used in measurement, control, and laboratory applications, including requirements for electromagnetic emissions and immunity, aiming to ensure that such equipment operates normally in electromagnetic environments while not causing harmful electromagnetic interference to the surrounding environment.

This standard applies to electrical equipment used in measurement, control, and laboratory applications, including various instruments, meters, sensors, actuators, etc. It provides manufacturers with a basis for design and testing to ensure that the equipment meets EMC requirements, and offers users reference to select and use the equipment to ensure its reliability and stability in actual applications.
- Measurement Equipment: Instruments used to measure various physical quantities (e.g., temperature, humidity, pressure, displacement, speed, acceleration, etc.), as well as equipment for cheMICal composition analysis and electrical parameter measurement (e.g., voltage, current, resistance, capacitance, inductance, etc.). Common examples include thermometers, pressure gauges, multimeters, and spectrometers.
- Control Equipment: Includes controllers, PLCs (programmable logic controllers), DCSs (distributed control systems), and various control devices for tasks such as process control, motor control, and temperature control in industrial automation systems. These devices adjust and control the controlled objects by receiving signals from sensors to achieve specific process requirements or production goals.
- Laboratory Equipment: Covers instruments and equipment used for various experiments and analyses in laboratories, such as electronic balances, centrifuges, oscillators, incubators, microscopes, chromatographs, mass spectrometers, etc. These devices are widely used in research, teaching, and quality testing laboratories for sample preparation, analysis, detection, and research.
GB/T 18268.26-2010
- Standard Name: "Electromagnetic Compatibility Requirements for Electrical Equipment for Measurement, Control, and Laboratory Use - Part 26: Special Requirements for In Vitro Diagnostic (IVD) Medical Devices".
- Scope: Specifically applies to in vitro diagnostic medical devices, which are used to diagnose diseases or other purposes, including determining health conditions for treatment, pain relief, cure, or disease prevention. These devices are primarily used to collect, prepare, and examine samples taken from the human body.
- Main Content: Based on GB/T 18268.1-2010, it specifies the basic requirements for the EMC immunity and emissions of such devices, considering the characteristics and electromagnetic environment of in vitro diagnostic medical devices. Tests are conducted according to the GB/T 17626 series of basic standards, with clear testing conditions and requirements for items like electrostatic discharge, radiated electromagnetic fields, power frequency magnetic fields, voltage dips, voltage interruptions, burst pulses, surge tests, and RF conduction.
Main Differences between GB/T 18268.1-2010 and GB/T 18268.26-2010:
- Scope
- GB/T 18268.1-2010: Applicable to electrical equipment used in professional, industrial, manufacturing, and educational settings, including devices and computing equipment for both industrial and non-industrial locations, covering measurement and testing, control, and laboratory equipment.
- GB/T 18268.26-2010: Specifically applies to in vitro diagnostic medical devices used for diagnosing diseases or other purposes, including determining health conditions for treatment, pain relief, cure, or disease prevention. These devices are primarily used to collect, prepare, and examine samples taken from the human body.
- Consideration of Equipment Characteristics
- GB/T 18268.1-2010: Considers the EMC of various general measurement, control, and laboratory electrical equipment. The equipment types are diverse, and the EMC requirements are general and universal.
- GB/T 18268.26-2010: Based on the characteristics of in vitro diagnostic medical devices and their electromagnetic environment, special EMC requirements are specified for these devices. For example, in vitro diagnostic medical devices may be more sensitive to electromagnetic interference because the accuracy of their test results is critical for disease diagnosis, so the standard may impose stricter immunity requirements.
- Test Requirement Differences
- GB/T 18268.1-2010: Specifies general EMC immunity and emissions requirements for electrical equipment, such as pulse groups, surges, conducted immunity, voltage dips, and interruptions for AC power ports, as well as pulse groups, surges, and conducted immunity for I/O signal and control ports. The specific test parameters and limits are based on the general usage environment and characteristics of the equipment.
- GB/T 18268.26-2010: In terms of immunity and emission requirements, the standard refines and specifies testing parameters and limits based on the actual usage and risk assessment of in vitro diagnostic medical devices. For example, stricter immunity requirements may apply to key diagnostic equipment in areas such as electrostatic discharge, radiated electromagnetic fields, and power frequency magnetic fields to ensure the device can work stably and accurately in complex medical environments, avoiding deviations in test results due to electromagnetic interference. There may also be special emission requirements to prevent interference with other medical devices or electronic instruments in the surrounding environment.
Email:hello@jjrlab.com
Write your message here and send it to us
 European Toy Safety Standard EN 71-20:2025
European Toy Safety Standard EN 71-20:2025
 EN 18031 Certification for Connected Devices on Am
EN 18031 Certification for Connected Devices on Am
 Compliance Guide for Portable Batteries on Amazon
Compliance Guide for Portable Batteries on Amazon
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
 LFGB Certification Cost and Timeline Guide
LFGB Certification Cost and Timeline Guide
 Bluetooth FCC Test Report
Bluetooth FCC Test Report
 Is FCC Testing Required?
Is FCC Testing Required?
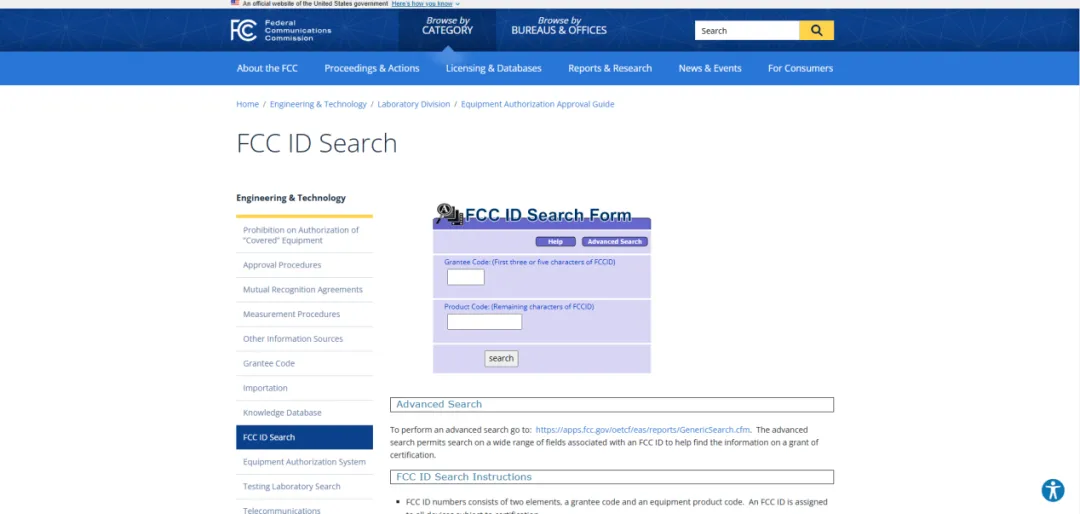 Where to Find FCC Test Reports
Where to Find FCC Test Reports
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




