
How to get an ASTM F2100:2025 Test Report?
In May 2025, the American Society for Testing and Materials (ASTM) officially released the latest version of its core standard for medical face masks—ASTM F2100:2025. This marks a significant update for manufacturers exporting medical masks to the U.S., introducing new testing requirements.
As an ISO 17025-accREDited international laboratory recognized by IAS (International Accreditation Service), JJR Laboratory in Chinaresponded promptly and has successfULly upgraded its testing capabilities to meet the new standard. We are now offering globally recognized testing servicesunder the ASTM F2100:2025 standard—helping your products comply with the new regulations and enter the U.S. market without delay.
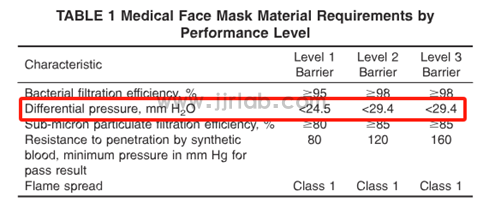
Key Changes in the New Standard Focus on "Differential Pressure" (ΔP) Testing:
Test Method Update:
The differential pressure (ΔP) test method has been revised from the previous version and is now standardized to EN 14683:2025 Annex C, one of the internationally recognized standards for medical mask testing.
Acceptance Limits Revised:
The acceptable ΔP limits for Level 1, Level 2, and Level 3 masks have been adjusted. This includes changes in both numerical values and measurement units.
> For the latest requirement details, please refer to the table below.
Additional Updates to Key Performance testing Standards:
Bacterial Filtration Efficiency (BFE):
Updated to ASTM F2101:2025
Particulate Filtration Efficiency (PFE):
Updated to ASTM F3502:2025
JJR Laboratory – Your Trusted Partner for the New Standard
We are fully equipped with the complete testing capabilities required under ASTM F2100:2025and its referenced standards, including EN 14683:2025 Annex C, ASTM F2101:2025, and ASTM F3502:2025.
All reports issued include the IAS-ILAC international mutual recognition mark, ensuring global recognitionof your testing results.
Let me know if you’d like the table mentioned ("latest requirement details") to be created or translated as well.
Email:hello@jjrlab.com
Write your message here and send it to us
 Irish Battery Act Requires an Authorised Represent
Irish Battery Act Requires an Authorised Represent
 Swedish Battery Act Requires an Authorised Represe
Swedish Battery Act Requires an Authorised Represe
 Amazon TIC Provider
Amazon TIC Provider
 Amazon Battery and Charger Requirements
Amazon Battery and Charger Requirements
 Amazon Japan METI A Domestic Administrator Service
Amazon Japan METI A Domestic Administrator Service
 What is "Amazon Japan PSE: A Domestic Adminis
What is "Amazon Japan PSE: A Domestic Adminis
 What Does "ASTM F963-17 Certified" Mean?
What Does "ASTM F963-17 Certified" Mean?
 ASTM F963 Board Games Compliance Testing
ASTM F963 Board Games Compliance Testing
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




