
IEC 60601-1-2 EMC Test for Medical Electrical Equipment
Comparison Between iec 60601-1-2 Edition 4 and Edition 3:
- RegULations and EMC standards for medical devices
- Key changes from Edition 3 to Edition 4
- Equipment labeling and documentation
- emi testing requirements
- Classification of electromagnetic environments
- Immunity testing requirements

EMC Standards for Medical Equipment
1. European Union (EU)
- Medical Devices: Regulated by Directive 93/42/EEC
Applicable Standards: EN 60601-1-2, EN 60601-2-x
- In Vitro Diagnostic (IVD) Devices: Regulated by Directive 98/79/EC
Applicable Standards: EN 61326-1, EN 61326-2-6
2. United States
- All Medical Equipment: Subject to fcc part 15 Subpart B or Part 18
Compliance via Verification, Declaration of Conformity (DoC), or Certification
Testing Standards: ANSI C63.4-2003 (for Part 15), FCC/OST MP-5 (for Part 18)
3. Canada
- All Medical Equipment: Requires test reports
Testing Standards: ICES-003, ICES-001
4. International Electrotechnical Commission (IEC)
- Medical Devices: IEC 60601-1-2, IEC 60601-2-x
- IVD Equipment: iec 61326-1, IEC 61326-2-6
Additional Requirements for Equipment with Radio Functions
1. EU: Regulated by R&TTE Directive 1999/5/EC; self-certification or notified body assessment required.
Example for Bluetooth (2.4 GHz): EN 300328, EN 301489-1/-17, en 62479
2. USA: RF devices are regulated under FCC Part 15 Subpart C; requires certification.
Testing Standard: ANSI C63.4-2003
3. Canada: Radio products must be certified by Industry Canada unless exempt.
Applicable Standards: RSS-GEN, RSS-210, RSS-310 (for Category I low-power devices)
emc testing Standards for Medical Equipment
- IEC/EN 60601-1-2:
Title: Medical electrical equipment – Part 1-2: General requirements for basic safety and essential performance – Collateral standard: Electromagnetic compatibility – Requirements and tests
Editions: 3rd (2007), 4th (2014)
- For IVD Equipment:
IEC/EN 61326-1: General EMC requirements
IEC/EN 61326-2-6: Specific to IVD medical equipment
Latest IEC editions: IEC 61326-1:2012, IEC 61326-2-6:2012
Latest EN editions: EN 61326-1:2013, EN 61326-2-6:2013
Key Changes in Edition 4 of IEC 60601-1-2
- Improved alignment with IEC 60601-1-11: clearer classification of use environments (professional, home healthcare, special environments)
- Introduction of higher RF immunity test levels due to proximity of mobile communications
- Enhanced risk management integration for EMC
- Removed the concept of "life-supporting"
- New methods to determine immunity levels for special environments and performance pass/fail criteria
Change in Title
- Edition 3: "... Electromagnetic compatibility – Requirements and tests"
- Edition 4: "... Electromagnetic disturbances – Requirements and tests"
- Now defined as a safety standard
Standard Objectives
- Emission standards aim to protect:
- Safety services
- Other medical devices and systems
- Non-medical systems such as computers
- Communication services (radio, TV, navigation)
- Immunity standards ensure:
- Continued basic safety and essential performance under EMC conditions throughout the equipment’s lifecycle
Key Definitions
- EMC (Electromagnetic Compatibility): Ability of the device/system to function reliably in its electromagnetic environment without causing unacceptable disturbance
- Electromagnetic Disturbance: Any EM phenomenon that could degrade performance
- Electromagnetic Emission: Radiation or conduction of electromagnetic energy
- Immunity: Resistance to performance degradation under EM disturbance
- Large Medical Equipment/System: Cannot fit within a 2m x 2m x 2.5m volume
Chapter 4: General Requirements
- 4.1: Reasonably foreseeable risks due to EM disturbances must be considered in risk management
- 4.2: Non-medical equipment used within systems must also meet relevant standards if they impact safety/performance
- 4.3: General test configuration should reflect worst-case scenarios and follow detailed guidelines (e.g., full cable/load setup, grounded chassis)
Input Voltage and Frequency for Testing
Each EMC test references specific input voltage and frequency guidelines. For example:
- Harmonic Current Emissions (IEC 61000-3-2): AC 230V single-phase, AC 400V three-phase, at 50/60Hz
- Voltage Fluctuations and Flicker (IEC 61000-3-3): Same voltage as above, 50Hz
- Radiated/Conducted Emissions (CISPR 11): Any voltage/frequency
- Immunity Tests (IEC 61000-4-2 to 4-11): Various combinations per test type
Chapter 5: Equipment Labeling and Documentation
- 5.1: Labeling for shielded-use equipment
- 5.2: Instructions for Use (IFU) must include:
- Environment declarations (including unsuitable environments like MRI rooms)
- Description of essential performance
- Warnings against stacked/adjacent equipment use
- Lists of user-replaceable components affecting EMC
- RF communications warnings (e.g., portable RF devices must be kept ≥30 cm away)
- 5.2.2: Technical descriptions must include:
- Compliance details for emissions/immunity
- Any deviations or relaxations from the standard
- Information for maintaining basic safety/performance
- For shielded locations: RF shielding performance, filtering requirements, and usage warnings
- For RF receiving/transmitting devices: frequencies, bandwidths, ERP, and modulation
- For large permanently installed systems: statement of exemption from full 80 MHz–6 GHz testing, warning of limited immunity
Chapter 6: Test Documentation
- 6.1: Test documentation must provide all relevant testing and planning information
- 6.2: Test plans must be detailed and submitted before testing
- 6.3: Test reports must comply with Chapter 9 of the standard
emi testing Overview
- Continuous Disturbances:
- Conducted Emissions: CISPR 14-1 / CISPR 11
- Radiated Emissions: CISPR 14-1 / CISPR 11
- Harmonics: IEC 61000-3-2
- Flicker: IEC 61000-3-3
- Discontinuous Disturbances:
- Clicks: CISPR 14-1 / CISPR 11
EMS Testing Overview
- Transient Phenomena:
- ESD: IEC 61000-4-2
- EFT/Burst: IEC 61000-4-4
- Surge: IEC 61000-4-5
- Voltage Dips/Interruptions: IEC 61000-4-11
- Vehicle Transients: ISO 7637-2 (vehicle use only)
- Steady-State Phenomena:
- Radiated RF: IEC 61000-4-3
- Conducted RF: IEC 61000-4-6
- RF Near Field from Wireless: IEC 61000-4-3 (temporary method)
Email:hello@jjrlab.com
Write your message here and send it to us
 European Toy Safety Standard EN 71-20:2025
European Toy Safety Standard EN 71-20:2025
 EN 18031 Certification for Connected Devices on Am
EN 18031 Certification for Connected Devices on Am
 Compliance Guide for Portable Batteries on Amazon
Compliance Guide for Portable Batteries on Amazon
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
 LFGB Certification Cost and Timeline Guide
LFGB Certification Cost and Timeline Guide
 Bluetooth FCC Test Report
Bluetooth FCC Test Report
 Is FCC Testing Required?
Is FCC Testing Required?
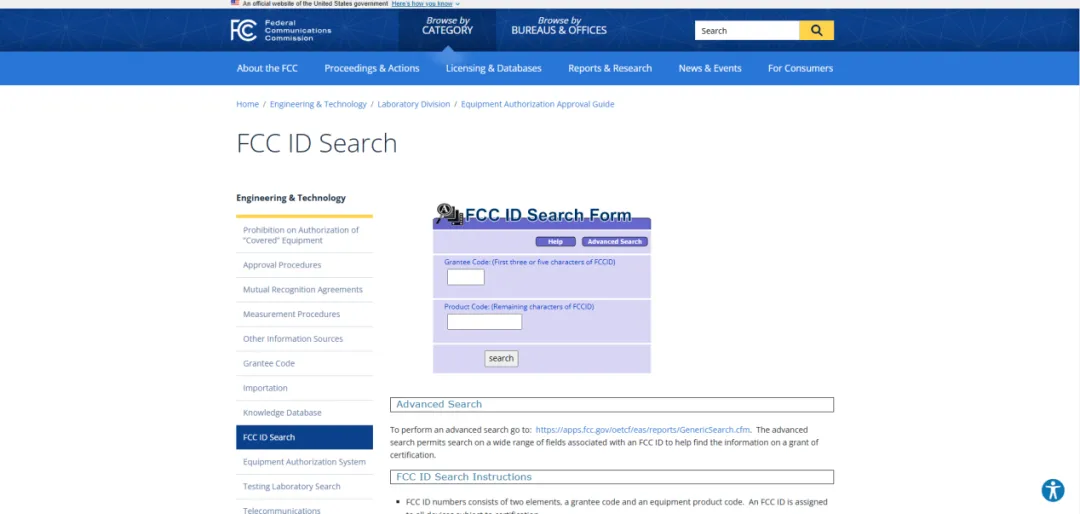 Where to Find FCC Test Reports
Where to Find FCC Test Reports
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




