
JJRLAB Showcases Medical Device Testing at CMEF Expo
On April 11th, the 89th China International Medical Equipment Fair opened grandly in Shanghai. As a benchmark for medical device quality technology services, JJRLAB showcased its distinctive testing services at booth 7.1B11, providing face-to-face, one-on-one consULtation services on medical device technology for medical device manufacturers.

1. Hearing aids
- GB/T 14199-2010
- GB/T 25102.100-2010
- GB/T 25102.2-2010
- GB/T 25102.7-2017
- GB/T 25102.13-2010
2. Ventilators
- GB 9706.212-2020
- YY 9706.270-2021
- YY 9706.272-2021
- YY 9706.274-2022
- YY 9706.279-2023
- YY 9706.280-2023
- YY 9706.284-2023
- GB 9706.290-2022
- YY/T 1778.1-2021
- iso 18562-1:2017
- ISO 18562-2:2017
- ISO 18562-3:2017
- ISO 18562-4:2017
3. Respiratory gas pathways
- YY/T 1778.1-2021
- ISO 18562-1:2017
- ISO 18562-2:2017
- ISO 18562-3:2017
- ISO 18562-4:2017
4. Pulmonary function testing equipment
- YY/T 1438-2016
- ISO 26782:2009
5. External defibrillators
- GB 9706.204-2022
6. Ultrasound therapy devices
- GB 9706.205-2020
- YY/T 1090-2018
- YY 0830-2011
- YY/T 1750-2020
7. Radiofrequency beauty instruments
- GB 9706.202-2021
8. Nebulizers
- YY/T 1743-2021
- ISO 27427:2013
- EN ISO 27427:2019
9. Rehabilitation equipment
- GB 24436-2009
- GB/T 37704-2019
- YY/T 0997-2015
- YY/T 0997-2015
- YY/T 0900-2013
10. OphthalMIC products
- YY 9706.258-2022
- YY 0065-2016
- YY/T 0634-2022
- YY 0599-2015
- ISO 15004-2:2007
11. Wound dressings
- YY/T 1627-2018
- YY/T 1477.5-2020
- YY/T 1477.6-2020
- YY/T 1849-2022
- YY/T 1283-2016
- YY/T 1511-2017
- YY/T 0308-2015
12. Neurological and cardiovascular surgical instruments
- YY 0285.1-2017
- YY 0285.3-2017
- YY 0285.4-2017
- YY/T 0285.5-2018
- YY/T 0285.6-2020
- YY 0450.1-2020
- YY/T 1660-2019
13. Obstetrics, auxiliary reproductive, and contraceptive instruments
- YY 0336-2020
- GB/T 7544-2019
- YY/T 1769-2022
- YY/T 1718-2020
Research testing
14. Cleaning, disinfection, and sterilization
- AAMI ST98:2022
- AAMI TIR12-2020
- AAMI TIR30-2011(R2017)
- ISO 17664-1:2021
- WS 310.1-2016
- WS 310.2-2016
- WS 310.3-2016
- YY/T 0734.1-2009
15. Network security
- GB/T 25000.51-2010 System and software engineering - Systems and software Quality Requirements and Evaluation (SQuaRE) - Part 51: Quality requirements and testing specifications for ready-to-use software product (RUSP).
16. Usability assessment reports
- YY/T 9706.106-2021 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: usability."
Email:hello@jjrlab.com
Write your message here and send it to us
 JJRLAB's New Chemical Laboratory Expansion Complet
JJRLAB's New Chemical Laboratory Expansion Complet
 JJRLAB 5G NR and Sub 6G communication testing
JJRLAB 5G NR and Sub 6G communication testing
 JJRLAB Completes Expansion of CPSC Full Project
JJRLAB Completes Expansion of CPSC Full Project
 What certifications for Middle East exports?
What certifications for Middle East exports?
 US-bound button cell batteries need UL4200A-2023
US-bound button cell batteries need UL4200A-2023
 Button batteries ANSI/UL 4200A-2023 Laboratory
Button batteries ANSI/UL 4200A-2023 Laboratory
 What is a Dun & Bradstreet (D-U-N-S®) Number?
What is a Dun & Bradstreet (D-U-N-S®) Number?
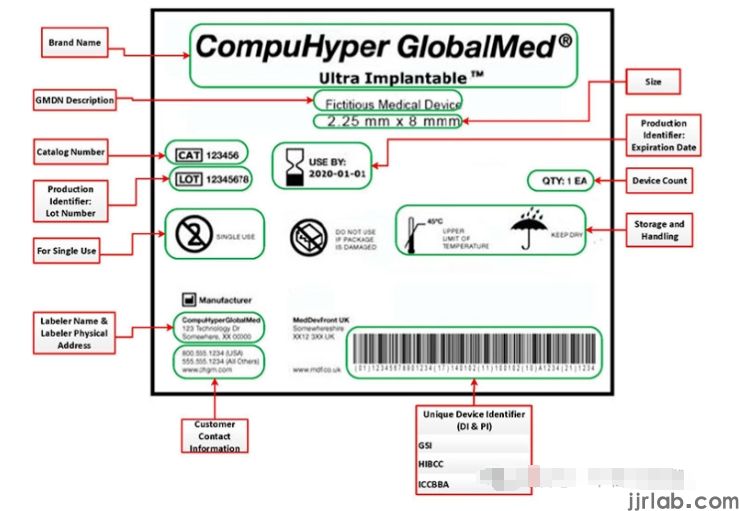 The Compliance Process of UDI with the U.S. FDA
The Compliance Process of UDI with the U.S. FDA
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




