
Recognition for Guangdong's Medical Device Testing Center
Recently, the Department of Science and Technology of Guangdong Province announced the list of engineering technology research centers for the year 2023. JJRLAB, after rigorous selection processes, was recognized as the "Guangdong Province Active Medical Device Performance Inspection and Testing Engineering Technology Research Center." This recognition follows their previous achievement of being designated as a "National High-tech Enterprise," further affirming JJRLAB's research and innovation capabilities officially.

The Active Medical Device Performance Inspection and Testing Engineering Technology Research Center at JJRLAB possesses state-of-the-art testing equipment and highly skilled engineers. It has obtained comprehensive qualifications and is committed to providing effective support for the sustainable development of upstream and downstream enterprises. Its aim is to accelerate the development and growth of the active medical device industry in Guangdong Province, streamline enterprise costs, enhance technological competitiveness, meet certification requirements from various countries, eliminate trade barriers in developed countries, and assist Shenzhen enterprises in expanding globally, thereby contributing to the development of the active medical device industry.
The establishment of a comprehensive Active Medical Device testing Engineering Technology Research Center, with the principles of high-quality, efficient, and modern services, aims to provide enterprises with one-stop market access technical services. By shortening product launch cycles, improving product quality, ensuring product safety, continuously optimizing technological innovation, and setting new benchmarks for industrial strength, it aims to build a high-level modern service system and promote high-quality development of enterprises and the entire medical device industry. In addition to serving medical device manufacturers, it also provides technical support for related industries in Guangdong Province, ULtimately creating a broad and impactful network of services, which holds significant and far-REACHing implications.
This recognition fully demonstrates the high recognition of JJRLAB's innovation capabilities and development potential by relevant departments in Guangdong Province. In the future, JJRLAB will continue to strengthen its core technology, enhance innovation in medical device inspection and testing-related technologies, and provide customers with more efficient, intelligent, and valuable services, contributing to technological innovation in the precise testing field of the Guangdong-Hong Kong-Macao Greater Bay Area.
Email:hello@jjrlab.com
Write your message here and send it to us
 JJRLAB's New Chemical Laboratory Expansion Complet
JJRLAB's New Chemical Laboratory Expansion Complet
 JJRLAB 5G NR and Sub 6G communication testing
JJRLAB 5G NR and Sub 6G communication testing
 JJRLAB Completes Expansion of CPSC Full Project
JJRLAB Completes Expansion of CPSC Full Project
 What certifications for Middle East exports?
What certifications for Middle East exports?
 US-bound button cell batteries need UL4200A-2023
US-bound button cell batteries need UL4200A-2023
 Button batteries ANSI/UL 4200A-2023 Laboratory
Button batteries ANSI/UL 4200A-2023 Laboratory
 What is a Dun & Bradstreet (D-U-N-S®) Number?
What is a Dun & Bradstreet (D-U-N-S®) Number?
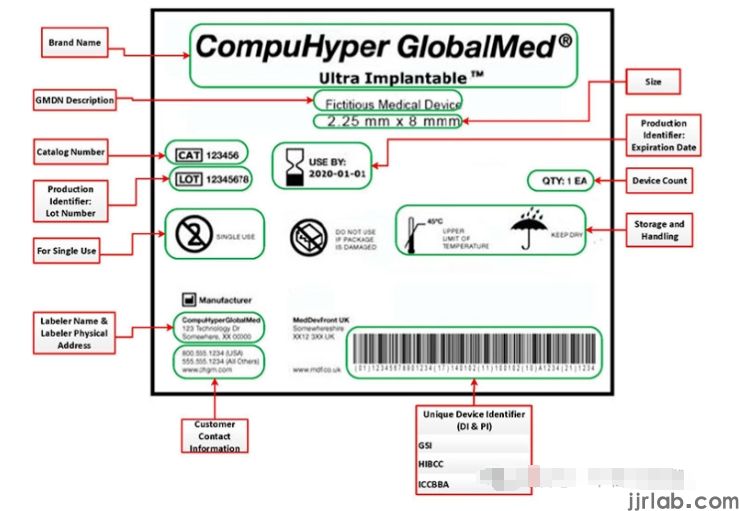 The Compliance Process of UDI with the U.S. FDA
The Compliance Process of UDI with the U.S. FDA
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




