
NMPA Certification and GB 9706 Testing for Dental Equipment
What is Dental Medical Equipment?
Dental equipment refers to a range of specialized tools and instruments used in dental treatment, examination, diagnosis, and surgery. These devices assist dentists in performing various treatments on teeth, gums, and the oral cavity.
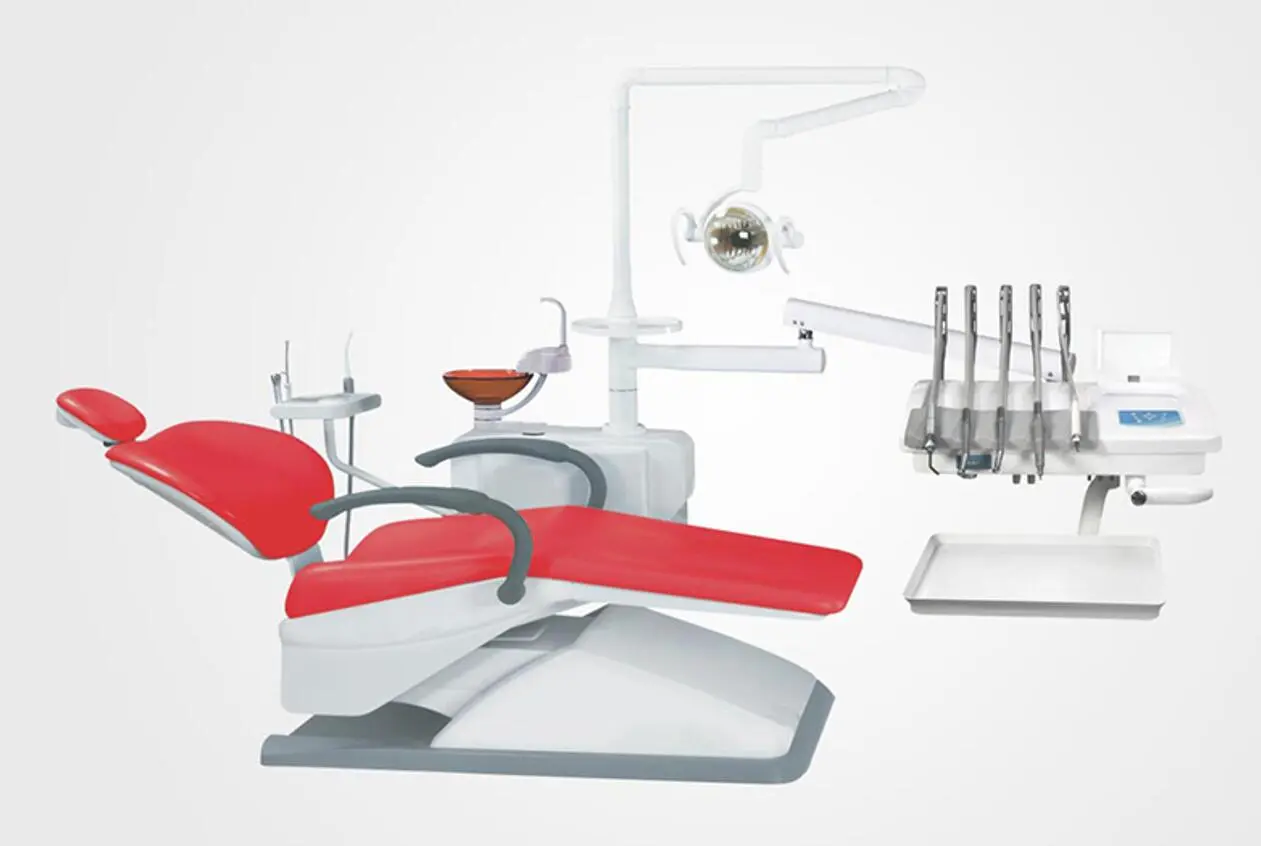
What are the types of Dental Medical Equipment?
Dental chair, dental handpiece, X-ray machine, dental probe, ULtrasonic scaler, dental curing light, saliva ejector, sterilization equipment, dental MICroscope, root canal treatment equipment, implant systems, dental imaging equipment.
Testing Standards for Dental Equipment in China
GB 9706.260-2020 Medical Electrical Equipment—Part 2-60: Particular requirements for the basic safety and essential performance of dental equipment
GB 9706.260 applies to the basic safety and essential performance requirements for dental units, dental patient chairs, dental hand-held devices, and oral lights (hereinafter collectively referRED to as dental equipment).
GB 9706.260 does not include amalgamators, sterilization equipment, and dental X-ray equipment.
Apart from the content specified in sections 7.2.13 and 8.4.1 of the general standard, the inherent hazards of the characteristics and functions of medical electrical equipment or systems covered by GB 9706.260 are not explained in the specific requirements of this section.
The purpose of GB 9706.260 is to establish requirements for the basic safety and essential performance of dental equipment.
GB 9706.263-2020 Medical Electrical Equipment—Part 2-63: Particular requirements for the basic safety and essential performance of dental extra-oral X-ray equipment
GB 9706.263 applies to the basic safety and essential performance (hereinafter referred to as ME equipment) of dental extra-oral X-ray equipment. The scope includes ME systems containing such ME equipment.
GB 9706.263 includes panoramic equipment, cephalometric equipment, and dental volumetric reconstruction equipment as defined in section 201.3.203.
The scope of GB 9706.263 is limited to X-ray equipment where the X-ray tube assembly includes high-voltage boundary components, and the geometric relationship between the X-ray source, the area of the patient being imaged, and the X-ray image receptor is pre-set and cannot be arbitrarily changed by the operator during use.
GB 9706.263 does not include intra-oral dental X-ray equipment.
The purpose of GB 9706.263 is to establish basic safety and essential performance requirements for ME equipment used in dental extra-oral X-ray imaging.
GB 9706.265-2021 Medical Electrical Equipment—Part 2-65: Particular requirements for the basic safety and essential performance of dental intra-oral X-ray equipment
GB 9706.265 applies to the basic safety and essential performance of intra-oral dental X-ray equipment and its main components, hereinafter referred to as ME equipment.
The scope of GB 9706.265 is limited to X-ray machines where the X-ray tube assembly contains high-voltage boundary components.
GB 9706.265 does not apply to extra-oral dental X-ray equipment.
The scope of GB 9706.265 does not include radiotherapy simulators, bone or tissue absorption densitometers, or dental fluoroscopic equipment.
The purpose of GB 9706.265 is to establish basic safety and essential performance requirements for ME equipment used in dental intra-oral X-ray imaging.
China JJR Laboratory provides GB 9706 testing services for dental medical devices, assisting enterprises in obtaining China's NMPA (CFDA certification) services.
Email:hello@jjrlab.com
Write your message here and send it to us
 Global Certification Guide for Lithium Batteries
Global Certification Guide for Lithium Batteries
 Compliance of Amazon 18650 Lithium Battery Product
Compliance of Amazon 18650 Lithium Battery Product
 What is CE Certification and EU Authorized Represe
What is CE Certification and EU Authorized Represe
 What Are the Lithium Battery Safety Tests?
What Are the Lithium Battery Safety Tests?
 What is the EN 61326-2-3 Standard?
What is the EN 61326-2-3 Standard?
 Why Do Smart Sockets Need IEC 60884 Certification?
Why Do Smart Sockets Need IEC 60884 Certification?
 Why Retest the Device if the 5G Module Already Has
Why Retest the Device if the 5G Module Already Has
 Overview of IEC 62087 Test Standard
Overview of IEC 62087 Test Standard
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




