
What is an EU Authorized Representative (EU-Rep)?
An eu authorized representative, also known as a European Representative (EU-Rep), acts on behalf of manufacturers outside the EU to ensure compliance with EU regULations.
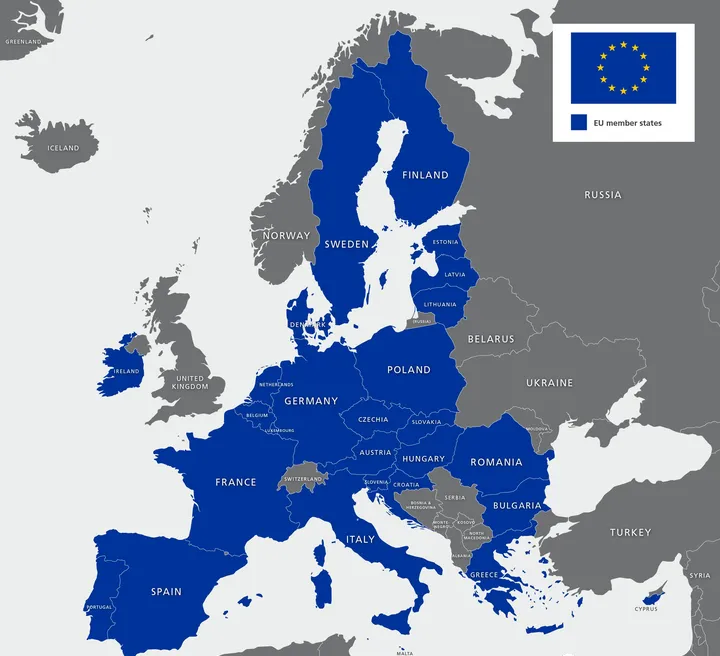
EU Regulations and the Role of an EU Authorized Representative
1. CE Certification Requirement: According to EU regulations, a manufacturer must have an agreement with an EU-Rep to apply for CE certification.
2. Product Labeling: After obtaining CE certification, the product packaging must display both the CE logo and the name and address of the EU-Rep. While the ce mark indicates the product's safety compliance, the EU-Rep ensures traceability and can be contacted by customers, customs, or EU authorities in case of any issues.
3. Technical Documentation (TCF): When applying for CE certification, manufacturers are requiRED to create a complete set of English technical documentation (TCF) covering risk analysis and essential requirement checks.
4. EU Import Requirements: Products marked with the CE logo imported from outside the EU must have the EU-Rep’s name and address clearly printed on the packaging, label, and user manual.
5. Maintenance of Technical Files: The EU-Rep must maintain up-to-date technical files for all products bearing the CE mark. These documents must be readily available for EU supervisory authorities and retained by the EU-Rep for at least five years after the final batch of products is marketed.
6. Incident Monitoring System: Manufacturers outside the EU are required to establish an effective incident monitoring system within the EU. The EU-Rep assists with accident reports, notifications, and product recalls.
7. Device Registration: The EU-Rep may handle registration with EU authorities on behalf of the manufacturer for medical devices.
8. Free Sale Certificate: The EU-Rep may also apply for a Free Sale Certificate on behalf of the manufacturer.
Process for Appointing an EU-Rep
1. Provide the ce certificate and report.
2. Complete the application form.
3. Sign the contract and make payment.
4. Receive the signed EU-Rep information.
Timeline
The process typically takes 3-5 business days.
Email:hello@jjrlab.com
Write your message here and send it to us
 Canada ISED Certification RSS-247 Standard Testing
Canada ISED Certification RSS-247 Standard Testing
 What Are the Product Compliance for Amazon Austral
What Are the Product Compliance for Amazon Austral
 Australia IoT Security Compliance
Australia IoT Security Compliance
 V16 Warning Light EU EN 18031 Cybersecurity Certif
V16 Warning Light EU EN 18031 Cybersecurity Certif
 Japan IoT Security JC-STAR Certification
Japan IoT Security JC-STAR Certification
 FCC SDoC Compliance Information Statement
FCC SDoC Compliance Information Statement
 What Does FCC SDoC Certification Mean?
What Does FCC SDoC Certification Mean?
 What is Bisphenol A (BPA) Testing?
What is Bisphenol A (BPA) Testing?
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




