
Active Medical Device Testing Laboratory
China JJR Laboratory is a national-level laboratory accREDited by the China National Accreditation Service for Conformity Assessment (CNAS). The laboratory is qualified for electromagnetic compatibility (EMC), safety, and performance testing of medical devices. We have established partnerships with notified bodies such as TÜV (Germany), Nemko, DNV (Norwegian Classification Society), UL, and Med-Cert.
Medical Device CE certification
CE-marked medical devices are closely related to user health and safety, and strict approval and market supervision requirements apply in various countries.
In the EU, relevant regulations mainly include Council Directive 93/42/EEC and 2007/43/EC (commonly known as the MDD directive), which apply to medical devices marketed in EU member states and certain non-EU countries.
CE Certification Categories and Example Products
1. Medical Device Directive (93/42/EEC & 2007/43/EC): medical gloves, thermometers, ECG machines, patient monitors
2. In Vitro Diagnostic Medical Device Directive (98/79/EC): blood/urine analyzers, biocheMICal analyzers, ELISA readers, blood glucose meters
3. Active Implantable Medical Device Directive (90/385/EEC): cardiac pacemakers
CE Product Categories and Certification Models
1. Class I: Self-declaration of conformity (e.g. examination gloves)
2. Class Is (Sterile): Self-declaration of conformity (e.g. umbilical clamps)
3. Class Im (Measuring): Design self-declaration + production audited by a notified body (e.g. thermometers)
4. Class IIa: Design self-declaration + production audited by a notified body (e.g. thermometers)
5. Class IIb: Design and production both audited by a notified body (e.g. patient monitors, ultrasound devices)
6. Class III: Design and production both audited by a notified body (e.g. cardiac pacemakers)
ISO 13485 System
ISO 13485:2016 is the quality management system for medical devices that addresses regulatory requirements.
It is generally considered a prerequisite for compliance with EU medical device management requirements.
Applicable to: medical device manufacturers, component suppliers, subcontractors, and distributors.
Testing Standards
Medical Devices (MDD)
1. EMC: IEC/EN 60601-1-2
2. Safety: IEC/EN 60601-1
In Vitro Diagnostic Devices (IVDD)
1. EMC: IEC/EN 61326-1, IEC/EN 61326-2-6
2. Safety: IEC/EN 61010-1, IEC/EN 61010-2-101, IEC/EN 61010-2-081
Example Harmonized Standards
1. ECG devices: iec 60601-2-27, IEC 60601-2-25, IEC 60601-2-47
2. Thermometers: ISO 80601-2-56, EN 12470 series, ASTM E1112-00, ASTM E1965-98
3. Pulse oximeters: ISO 80601-2-61
4. Blood pressure devices: IEC 80601-2-30, ISO 81060-1/2, IEC 60601-2-34
5. Multi-parameter monitors: IEC 60601-2-49
6. Infusion pumps: IEC 60601-2-24
7. Laser devices: IEC 60601-2-22
8. Medical beds: IEC 60601-2-52
9. Others: IEC 60601-1-8, IEC 60601-1-11, IEC 60601-2-10, etc.
> We update our CNAS scope of accreditation in accordance with the latest standard versions.
Our Services
1. Medical device product testing
2. ISO 13485 system consulting
3. EU representative recommendation
4. TCF technical documentation guidance and preparation
FDA Registration
The U.S. Food and Drug Administration (FDA) regulates medical devices through its Center for Devices and Radiological Health (CDRH).
FDA Medical Device Classification and Registration Models
1. Class I: General controls, 510(k)-exempt or special controls (e.g. lenses, canes), online registration and number assignment
2. Class II: Special controls 510(k) or general controls (e.g. dental devices, surgical gloves, electric wheelchairs), requires submission of 510(k) premarket notification
3. Class III: Premarket approval (PMA) or special controls 510(k) (e.g. cardiac pacemakers)
All companies must complete establishment registration and product listing.
Our FDA Services
1. Develop and provide testing programs
2. Assist in preparing 510(k) documentation and materials
3. Draft 510(k) files
4. Recommend U.S. agent services
5. Assist with establishment registration and product listing
6. Facilitate communication with designated review bodies
Email:hello@jjrlab.com
Write your message here and send it to us
 European Toy Safety Standard EN 71-20:2025
European Toy Safety Standard EN 71-20:2025
 EN 18031 Certification for Connected Devices on Am
EN 18031 Certification for Connected Devices on Am
 Compliance Guide for Portable Batteries on Amazon
Compliance Guide for Portable Batteries on Amazon
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
 LFGB Certification Cost and Timeline Guide
LFGB Certification Cost and Timeline Guide
 Bluetooth FCC Test Report
Bluetooth FCC Test Report
 Is FCC Testing Required?
Is FCC Testing Required?
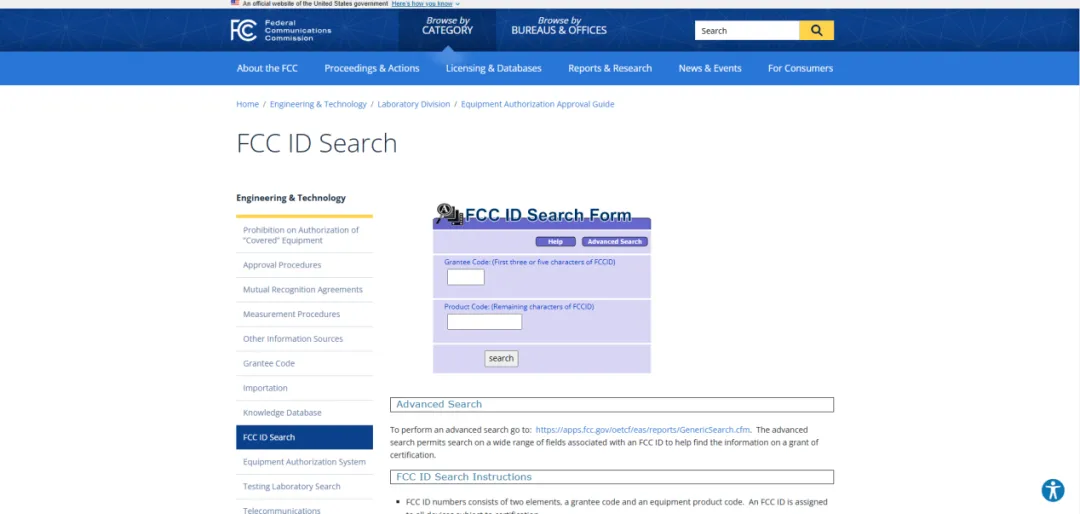 Where to Find FCC Test Reports
Where to Find FCC Test Reports
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




