
Introduction to METI Registration in Japan
The Ministry of Economy, Trade and Industry (METI) of Japan has released detailed information regarding this method, developed by the Commerce and Information Policy Bureau's Information Economy Division. The PSE product meti registration requires compliance with Japan's DENTORL law (Electrical Appliances and Materials Control Law), mandating that Japanese purchasers must register and declare their products to METI within one month of acquisition. Additionally, the purchaser's name or ID must be marked on the product for future sales monitoring. In other words, electronic products must be METI registeRED to be sold in Japan.
pse certification
1. PSE Certification: This is Japan's mandatory safety certification that verifies electrical and electronic products have passed safety standards testing under the DENAN Law or international IEC standards.
2. Under the DENTORL law, products entering the Japanese market must pass PSE safety certification. Among these, 116 types of Class A products require a diamond-shaped pse mark, while 341 types of Class B products need a circULar PSE mark.
3. All products within the PSE certification scope must be registered with METI by Japanese purchasers within one month of purchase to obtain approval documentation.
Registration Requirements
1. Notification of Business: This declaration by the Japanese importer includes manufacturer information, product details, and signing date.
2. Approval Documentation: The approval certificate issued by METI requires that the importer presents the PSE supplementary certificate to METI for registration.
3. Japanese purchasers must register with METI within one month of acquiring products, and only those with registered Japanese trading companies can apply for registration.
4. METI registration is divided into two types:
1) With importer qualifications;
2) Without importer qualifications (importer qualification can be leased).
Declaration Process
1. The applicant submits the application documents.
2. Testing reports and relevant documents are submitted to METI.
3. Application documents are registered.
4. Product label design and confirmation.
Documentation Checklist
1. Application form
2. PSE testing report (products with batteries or adapters also require their own PSE testing reports)
3. Product manual (in Japanese and English)
4. Product specification sheet (BOM, product photos)
5. Statement of differences (if there are series models in the report)
Email:hello@jjrlab.com
Write your message here and send it to us
 What is Protection Class EN 60529?
What is Protection Class EN 60529?
 IP69 Certified Protection
IP69 Certified Protection
 California Energy Commission Testing Lab
California Energy Commission Testing Lab
 What Does the Canadian IC Mark Mean?
What Does the Canadian IC Mark Mean?
 How Much is the Canada IC ID Certification cost?
How Much is the Canada IC ID Certification cost?
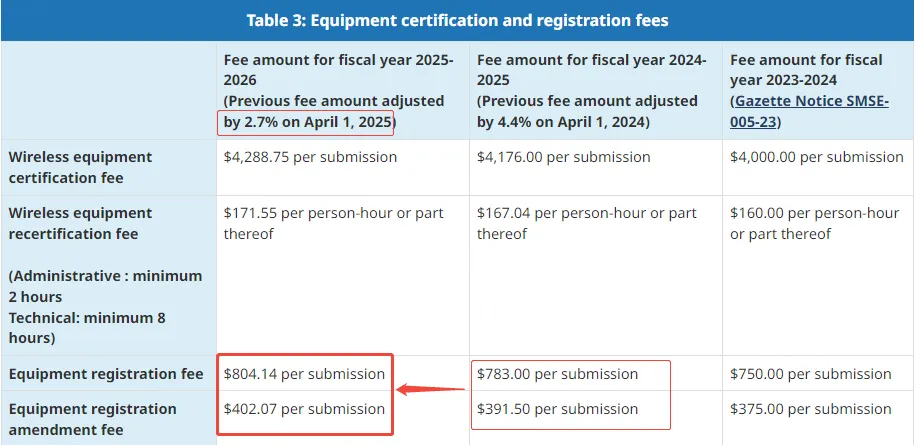 How Much is the Canada IC ID Certification Fee?
How Much is the Canada IC ID Certification Fee?
 How Much is the UL 982 Test Report csost?
How Much is the UL 982 Test Report csost?
 ISO/IEC 17025 Accredited Test Laboratory
ISO/IEC 17025 Accredited Test Laboratory
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




