Medical Device Intracutaneous Reactivity Test Laboratory
Intracutaneous Reactivity Test Introduction
There may be a variety of cheMICal components in the final medical device, such as various additives, including slippers, dyes, adhesives, and sterilant residues produced during the sterilization process, and whether these components have potential irritation activity shoULd be consideRED. The characteristics of irritation will manifest as inflammation, redness, swelling, heat and pain. Therefore, irritation testing is essential to evaluate the potential of medical devices to cause irritation reactions immediately after exposure to the human body.
Irritation tests include intradermal reaction, skin irritation, eye irritation, and mucosal irritation tests. When the device is not suitable for the use of the device (eyes, skin or mucosa), such as implantable devices and external access devices, it is necessary to select intradermal reaction test. Intradermal reaction test is an in vivo test that evaluates the possibility of irritation by intradermally injecting the extract of the medical device.

2. Based on standards
2.1 iso 10993-23 Biological evaluation of medical devices – Part 23: Tests for irritation;
2.2 GB/T16886.10 Biological evaluation of medical devices – Part 10: Irritation and skin sensitization tests.
3. Test system
3 New Zealand rabbits.
4. Preparation of test samples
Polar and non-polar extracts were prepared according to GB/T 16886.12.
5. Test steps
5.1 Healthy adult New Zealand rabbits that had been acclimatized for 7 days were used. Four hours before the test, 10 cm × 15 cm of rabbit hair was cut off on both sides of the spine of three rabbits using an automatic shaver. The rabbit skin was not damaged during the hair cutting process. Rabbits with intact skin were selected for the test.
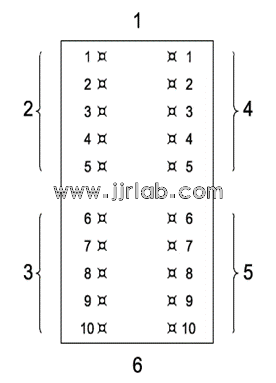
5.2 Five injection points were made at equal distances on the left and right sides of the spine of each rabbit. The polar extract was injected into the 5 points on the upper left side, the non-polar extract was injected into the 5 points on the lower left side, the polar control solution was injected into the 5 points on the upper right side, and the non-polar control solution was injected into the 5 points on the lower right side. The remaining rabbits were injected in the same way, and the injection volume at each injection point was 0.2 mL. As shown in Figure 1.
Figure 1 Location of intradermal injection points | 1. Headend 2. 0.2 mL polar extract injection point 3. 0.2 mL non-polar extract injection point 4. 0.2 mL polarity control solution injection point 5. 0.2 mL non-polar control solution injection point 6. Tail end |
After the injection, observe and record the skin reactions at each injection site immediately, 24 h, 48 h and 72 h later. Score the erythema and swelling at each injection point during each observation period according to Table 1, and record the test results.
6. Result evaluation
After scoring at 72h, add up all the erythema and edema scores of each animal's test sample or blank control at 24h, 48h, and 72h, and then divide by 15 (3 observation periods × 5 injection sites) to calculate the score of each animal's test sample or blank control. Add the scores of the three animals and divide by 3 to get the total average score of each test sample and the corresponding blank control. The final score of the test sample can be obtained by subtracting the blank control score from the test sample score. If the final score of the test sample is not greater than 1.0, it meets the test requirements. During any observation period, if the average reaction of the test sample is suspected to be greater than that of the blank control, 3 more rabbits should be retested. If the final score of the test sample is not greater than 1.0, it meets the test requirements. (Note: The blank control sample is a polar or non-polar solvent control.)
Table 1. Intradermal reaction scoring system
reaction | Scoring |
Erythema and eschar formation | |
No erythema | 0 |
Very slight erythema (barely visible) | 1 |
Clear erythema | 2 |
Moderate erythema | 3 |
Severe erythema (purple red) to eschar formation that cannot be graded | 4 |
Edema formation | |
No edema | 0 |
Very slight edema (barely visible) | 1 |
Clear edema (clear edge of swelling) | 2 |
Moderate edema (swelling about 1 mm) | 3 |
Severe edema (swelling greater than 1 mm beyond the contact area) | 4 |
Stimulate the highest score | 8 |
Note: Other abnormalities at the injection site should be recorded and reported | |
Email:hello@jjrlab.com
Write your message here and send it to us
 Canada ISED Certification RSS-247 Standard Testing
Canada ISED Certification RSS-247 Standard Testing
 What Are the Product Compliance for Amazon Austral
What Are the Product Compliance for Amazon Austral
 Australia IoT Security Compliance
Australia IoT Security Compliance
 V16 Warning Light EU EN 18031 Cybersecurity Certif
V16 Warning Light EU EN 18031 Cybersecurity Certif
 Japan IoT Security JC-STAR Certification
Japan IoT Security JC-STAR Certification
 FCC SDoC Compliance Information Statement
FCC SDoC Compliance Information Statement
 What Does FCC SDoC Certification Mean?
What Does FCC SDoC Certification Mean?
 What is Bisphenol A (BPA) Testing?
What is Bisphenol A (BPA) Testing?
Leave us a message
24-hour online customer service at any time to respond, so that you worry!