
How to get a D-U-N-S® Number for US FDA Registration?
When medical device enterprises apply for FDA registration and certification, they often come across a term — D-U-N-S® Number. Applying for a D-U-N-S® Number is a necessary prerequisite for enterprises to successfULly complete FDA registration and certification. This is because the number is requiRED for multiple procedures, including the establishment of a paid enterprise account and the application for a UDI database account.
Today, JJR LAB will provide a detailed introduction to the D-U-N-S® Number.

What is a D-U-N-S® Number?
The D-U-N-S® Number (short for Data Universal Numbering System) is a real-time dynaMIC enterprise identification code consisting of 9 digits. It is derived from the global coding system developed and managed by Dun & Bradstreet.
Widely used for enterprise identification and the organization and collation of business information, the D-U-N-S® Number enables quick identification and location of enterprise details, facilitating effective risk management.
Why Does the FDA Require a D-U-N-S® Number?
As a globally recognized enterprise identification code, the D-U-N-S® Number is unique — each business entity is assigned a one-to-one corresponding code that will never be reused, equivalent to an enterprise’s "ID card number".
① When applying for a D-U-N-S® Number, enterprises are required to submit information such as their name and address. The FDA can use this number to identify and verify enterprises, which helps improve the quality and accuracy of FDA registration data.
② The D-U-N-S® Number is closely linked to an enterprise’s credit evaluation. The official FDA can quickly and accurately check an enterprise’s historical records and credit standing through this number, ensuring product quality and safety.
D-U-N-S® Number Application Document Checklist
① Company name (in both Chinese and English)
② Company office address (in both Chinese and English)
③ Mailing address (if different from the office address)
④ Name and position of the company’s manager
⑤ Number of employees
⑥ Business contact person
⑦ Telephone number
⑧ Job title of the contact person
⑨ Email address
⑩ Type of business operation
⑪ Scanned copy of the business license
⑫ Signed and sealed enterprise authorization letter
JJR LAB’s Advantages
JJR LAB can help medical device enterprises quickly and accurately complete D-U-N-S® Number applications. We also provide a one-stop service for medical device FDA registration and certification, helping enterprises successfully enter the US market.
Email:hello@jjrlab.com
Write your message here and send it to us
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
 LFGB Certification Cost and Timeline Guide
LFGB Certification Cost and Timeline Guide
 Bluetooth FCC Test Report
Bluetooth FCC Test Report
 Is FCC Testing Required?
Is FCC Testing Required?
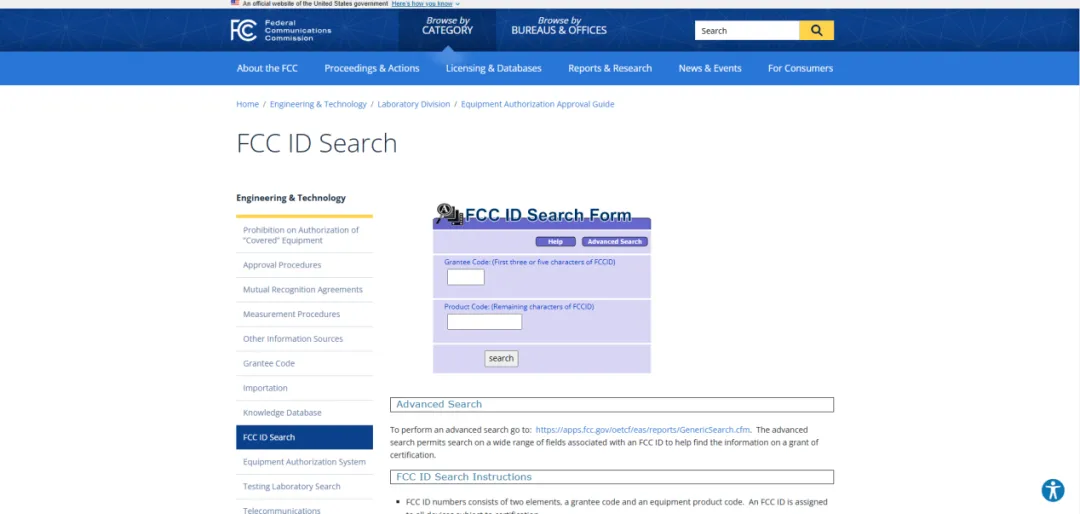 Where to Find FCC Test Reports
Where to Find FCC Test Reports
 LFGB Compliance Testing for Plastic Food Contact M
LFGB Compliance Testing for Plastic Food Contact M
 How to get LFGB Compliance Report for Food Grade P
How to get LFGB Compliance Report for Food Grade P
 LFGB Certification Process for Kitchenware Product
LFGB Certification Process for Kitchenware Product
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




