
Canada Vaping Labelling Packaging Regulations — SOR/2019-353
The current Canadian regULation “Vaping Products Labelling and Packaging Regulations” (SOR/2019-353) specifies requirements for the labelling and packaging of vaping products sold in Canada. The following provides a detailed interpretation of the latest provisions:
Scope of Application
- Applies to all vaping products and their packaging sold at retail in Canada.
- Does notapply to vaping products regulated under the Food and Drugs Act.
Nicotine Concentration Declaration
- Canada prohibits the sale of vaping products containing more than 20 mg/mLof nicotine (per Regulation SOR/2021-123).
- All nicotine-containing vaping products must display their nicotine concentration.
- The concentration must be preceded by “Nicotine —” and followed by the unit “mg/mL”.
Health Warnings for Nicotine-Containing Products
- Any vaping product containing nicotine must display a health warning.
- The wording “Health Canada” must appear next to or below the English health warning, and “Santé Canada” next to or below the French version.
Nicotine-Free Product Declaration
Only one of the following expressions may appear on a nicotine-free vaping product:
A. English: “Nicotine-free”; French: “Sans nicotine”.
B. English: “No nicotine”; French: “Aucune nicotine”.
C. English: “Does not contain nicotine”; French: “Ne contient pas de nicotine”.
Information Display Requirements
A. All requiRED health warnings and permitted statements must appear equally in both official languages.
B. Required information and permitted statements must be indelible and non-removable.
C. Integrity:Opening the product or packaging in a normal way must not cut, damage, or obscure any required text.
D. Clarity:
- Must use a standard sans-serif font, not compressed, extended, or decorated.
- Text must appear black on a white background, clearly legible, and remain legible for the product’s lifespan under normal conditions.
- Font height is measured based on uppercase or lowercase letters with ascenders or descenders (e.g., “b” or “p”).
E. All required text must use a uniform font and size.
F. Font size requirements for nicotine declaration or health warnings:
a. Main display panel ≥ 45 cm² → min. height 3 mm(8 pt).
b. Main display panel 10–45 cm² → min. height 2 mm(6 pt).
c. Main display panel < 10 cm² → min. height 1.5 mm(4.5 pt).
G. The first word of the health warning must be in bold uppercase letters, while the rest is in lowercase and not bold.
Completeness and Layout of Information
A. All required information must be positioned as close as possible to the top edgeof the label or package, with the nicotine concentration statement placed above the health warning, if applicable.
- Must be visible and readable without additional manipulation during normal sale or use.
- Font size must be at least 2 mm(6 pt).
B. The package must include a leafletshowing the required health warning.
C. Labels must not obscure, interfere with, or make illegible any required information, nor interfere with safe or normal product use.
Ingredient List Requirements
A. Every vaping product must provide an ingredient list, using common (non-abbreviated) names.
- Both English and French versions must be titled “Ingredients:”.
B. Ingredients added solely for flavor must be labeled as:
- “flavour” (English) and “arôme” (French).
- When multiple flavoring ingredients are combined, they must be listed according to their total concentration proportion.
C. The ingredient list must appear on the immediate containerand on any outer packaging, or be affixed as a labelto the device or component.
D. Ingredient ordering:
a. Ingredients ≥ 1% concentration → listed in descending order by proportion.
b. Ingredients < 1% → listed in any order, following (a).
- When concentration is expressed as a percentage, it refers to the weight-to-volume ratioof the ingredient.
Child-Resistant Packaging Requirements
A. The child-resistant container must only be openable or removable using a tool not suppliedwith the container, or comply with one of the following standards(or equivalent):
a. CAN/CSA Z76.1-16— Reclosable Child-Resistant Packages(Canada);
b. ISO 8317:2015— Child-Resistant Packaging – Requirements and Testing Procedures(International);
c. 16 CFR 1700.20— Testing Procedures for Special Packaging(United States).
B. The container must maintain its child-resistant propertiesthroughout its shelf life and, for refillable containers, under normal storage, sale, and use conditions.
Opening and Closing Instructions
A. Containers meeting child-resistant standards must display:
- “USE ENTIRE CONTENTS ON OPENING. THIS CONTAINER IS NOT CHILD-RESISTANT ONCE OPENED.”
- “UTILISER LA TOTALITÉ DU CONTENU APRÈS OUVERTURE. UNE FOIS OUVERT, LE CONTENANT N’EST PLUS UN CONTENANT PROTÈGE-ENFANTS.”
B. If the main display panelof the immediate container is smaller than 45 cm², the statement need not appear on it.
- If the outer packagemain display panel is smaller than 45 cm², the statement must appear on the label adjacent to the container.
C. For small containers without packaging:
- 15–45 cm² → statement on the container surface or attached label.
- ≤15 cm² → statement must appear on an attached label.
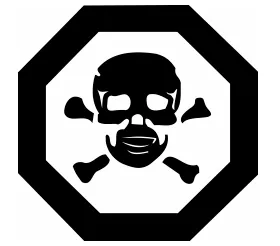
Toxicity Information
A. For vaping products with nicotine concentration ≥ 0.1 mg/mL, the immediate container must display:
- The hazard symbol(identical in size and design to the reference symbol).
- The bilingual toxicity warning and first aid statement:
- English: “POISON: if swallowed, call a Poison Control Centre or doctor immediately.”
- French: “POISON : en cas d’ingestion, appeler immédiatement un centre antipoison ou un médecin.”
B. The information must be displayed in both official languages, remain legible throughout the product’s life, and appear in black text on a white background.
C. When presented as text, it must use a standard sans-serif font, not compressed, extended, or decorated, with all characters uniform in font and size.

Display of Hazard Symbols
The toxicity warning and first aid statement must appear as follows:
a. The word “POISON” in bold uppercase letters.
b. The statement positioned next to or below the hazard symbol.
Email:hello@jjrlab.com
Write your message here and send it to us
 EMC Item – Introduction to Radiated Emission Test
EMC Item – Introduction to Radiated Emission Test
 IEC 62471 Photobiological Safety of Lamps and Lamp
IEC 62471 Photobiological Safety of Lamps and Lamp
 New European Toy Standard EN 71-1:2026
New European Toy Standard EN 71-1:2026
 EN71 Series Standards Compliance February 13, 2026
EN71 Series Standards Compliance February 13, 2026
 European Toy Safety Standard EN 71-20:2025
European Toy Safety Standard EN 71-20:2025
 EN 18031 Certification for Connected Devices on Am
EN 18031 Certification for Connected Devices on Am
 Compliance Guide for Portable Batteries on Amazon
Compliance Guide for Portable Batteries on Amazon
 2026 EU SVHC Candidate List (253 Substances)
2026 EU SVHC Candidate List (253 Substances)
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




