
What is Biocompatibility Testing for Medical Devices?
What is biocompatibility?
Biocompatibility refers to the various complex biological, physical, and chemical reactions that occur when biomaterials come into contact with specific parts of the body, determining whether certain materials or drugs are "compatible" with the human body upon contact or implantation, and whether they will cause harm to our bodies. There are numerous testing parameters, primarily including cytotoxicity, sensitization, irritation, systemic toxicity (acute toxicity), subacute toxicity (subacute toxicity), genetic toxicity, implantation, chronic toxicity, carcinogenicity, reproductive and developmental toxicity, and biodegradation.
ISO 10993 standards typically include testing parameters such as in vitro cytotoxicity testing, skin irritation testing, and sensitization tests, also known as the fundamental three of biological evaluation:
Cytotoxicity: Samples' extracts are cULtuRED in vigorously growing cell suspensions, and cell morphology is observed. (GB/T 16886.5-2003, iso 10993-5-1999)
Skin irritation: Samples' extracts are dripped onto absorbent gauze or directly applied to shaved areas on the backs of animals, and the reactions are observed. (GB/T 16886.10-2005, iso 10993-10)
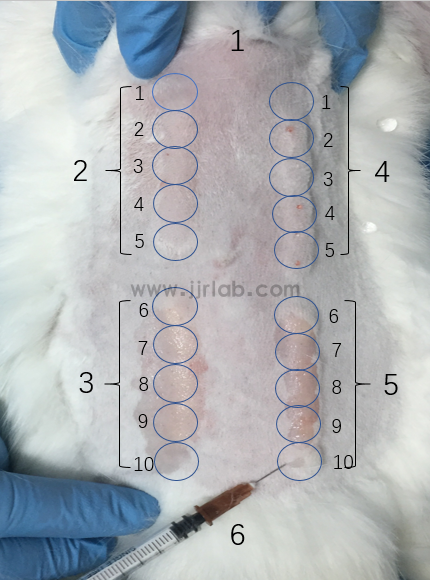
Sensitization test: Samples' extracts are intradermally injected into animal skin, followed by application of gel materials to the shaved backs of animals. After induction and challenge phases, corresponding sites are observed. (GB/T 16886.10-2005, ISO 10993-10)
In vitro cytotoxicity model:
1. Cells
Mouse fibroblast cells L929, NIH3T3; Human normal liver cells LO2.
2. Preparation of extracts
Gel materials + culture medium, 37°C, 72h, filtered through 0.22um filter after incubation.
3. Cell grouping (n=3)
① Normal cells
② Normal cells + extract concentration A
③ Normal cells + extract concentration B
④ Normal cells + extract concentration C
Intervention time: 24h, 48h, 72h; CCK-8 detects cytotoxicity.
Skin irritation test animal model:
1. Material preparation
① Experimental animals: Rabbits.
② Other materials: Gel, extract (gel material + culture medium, 37°C, 72h, filtered through 0.22um filter, dripped onto gauze for use).
2. Modeling method
① Shave the area on both sides of the spine of the back, incubate gel/extract in the depilated area on the left side, apply solvent/vehicle in the depilated area on the right side, remove the drug after 4h of bandaging and wash with warm water,
② Observe skin reactions and score according to erythema and edema reactions.
③ Subsequently, the same treatment is performed at the same time and in the same manner for 7 consecutive days, and skin reactions are observed and scored.
3. Evaluation criteria according to ISO 10993: Irritation and skin sensitization test:
① The primary irritation score for each group is the sum of the scores for each group divided by the number of test sites observed;
② The primary irritation score for each animal is the primary irritation score for the material group minus the primary irritation score for the control group;
③ Primarily Stimulation Index (PII) is the primary irritation score for each animal divided by the total number of animals.
4. Judgment of stimulus reaction type
① PII from 0.0 to 0.4 indicates a slight reaction;
② 0.5 to 1.9 indicates a mild reaction;
③ 2.0 to 4.9 indicates a moderate reaction;
④ 5.0 to 8.0 indicates a severe reaction.
Sensitization test animal model:
1. Material preparation
① Animals: Guinea pigs
② Drugs: Extract (gel material + culture medium, 37°C, 72h, filtered through 0.22um filter, PBS, TritonX-100).
2. Modeling method (combination of intradermal and topical application)
① Intradermal induction: Guinea pigs are randomly divided into 3 groups: test group, negative control group, and positive control group, with 6 animals per group. The back area of the guinea pigs is depilated 24h before the experiment. With the spine as the axis, three injection points are set on each side, and each point is intradermally injected with 0.1mL. The three different solutions A, B, and C are injected from top to bottom:
Test group (A) Freund's complete adjuvant/physiological saline 1:1
(B) Gel extract
(C) A solution/B solution 1:1
Positive control group (A) Freund's complete adjuvant/physiological saline 1:1
(B) 5% formaldehyde solution
(C) A solution/B solution 1:1
Negative control group (A) Freund's complete adjuvant/physiological saline 1:1
(B) Physiological saline
(C) A solution/B solution 1:1
After 7 days of injection, enter the local induction phase.
② Local induction: 24h before application, the back is depilated again, the skin is disinfected, and massage with 10% SDS is performed to introduce into the skin. On the second day, gauze soaked with gel extract, physiological saline, and 5% formaldehyde solution is respectively applied to the experimental group, negative control group, and positive control group areas on the backs of animals, and bandaged and fixed. After 48h, remove the bandage and gauze, and enter the challenge phase after 14 days.
③ Challenge phase: Soak the gauze in gel extract, negative control liquid, and positive control liquid, shave the abdomen of the animal, apply the gauze, and bandage and fix it. Remove the bandage and gauze after 24h. Then observe the skin changes at the stimulated site of each group of animals at 24h and 48h.
3. Magnusson and Kligman grading criteria:
① If the score of the negative control group animals is less than 1, and the score of the test group animals' skin is greater than or equal to 1, sensitization occurs.
② If the score of the negative control group animals is greater than or equal to 1, and the reaction of the test group animals exceeds the strongest reaction in the negative control group, sensitization occurs.
③ Sometimes, more animals in the test group have reactions than in the control group, but the intensity of the reaction does not exceed that of the control group. In this case, a second injection is needed 1 to 2 weeks after the initial sensitization to determine the cause of sensitization. The method used is the same as the initial infection, using the untested side of the animal.
Blood compatibility test:
1. Material preparation
① Sample: Fresh human anticoagulant whole blood
② Drugs: Extract (gel material + culture medium, 37°C, 72h, filtered through 0.22um filter, PBS, TritonX-100).
2. Testing method
① Prepare human citrated whole blood (9:1, whole blood:3.2% sodium citrate anticoagulant);
② Centrifuge the anticoagulated whole blood at 1000 rpm for 10 minutes, take the lower layer of red blood cells and add an equal volume of PBS for mixing;
③ Centrifuge at 1000 rpm for 10 minutes, take the lower layer of red blood cells and dilute with PBS to 5%;
④ Take 6mL of extract, PBS (negative control), 0.1% TritonX-100 (PBS dilution, positive control), add 4mL of red blood cell dilution solution to each, and mix gently;
⑤ After incubating at 37°C for 1 hour, centrifuge at 3000 rpm for 10 minutes;
⑥ Measure absorbance at 540 nm of the supernatant.
3. Hemolysis rate calculation formula
Hemolysis ratio (%) = [(As− A0) ⁄(Ap− A0)] × 100%
As is the absorbance of each group, A0 is the absorbance of the blank control group, and Ap is the absorbance of the positive control group.
Tissue compatibility test:
1. Experimental materials
① Animals: SD rats.
② Drugs: Gel.
2. Modeling method
After anesthesia of rats, gel is implanted into the back muscles of rats. Rats are sacrificed at 0, 7, and 14 days. Tissues around the gel are fixed for 48h, dehydrated and embedded in paraffin, sectioned, and stained with HE.
(Tissue immune compatibility optional: IHC detection of CD68 expression)
3. Pathological analysis
① If no obvious cell infiltration is observed in HE staining, it indicates good tissue compatibility of the material.
② If the number of CD68-positive cells in IHC staining is not significantly different from that in the control group, it indicates that the material does not trigger inflammation and has good in vivo immune compatibility.
Applications
Currently, research on the biocompatibility of gel materials involves multiple fields such as medicine, pharmacy, and bioengineering:
1. Medical biomaterials: In the medical field, gel materials are widely used in tissue engineering, repair, and regeneration, and biocompatibility is an important indicator to evaluate whether these gel materials cause toxicity or immune reactions when in contact with organisms.
2. Drug delivery systems: Gel materials can serve as carriers for drug delivery systems, used to control the release of drugs and improve their bioavailability. Biocompatibility studies in this context help evaluate the interaction of gel materials with drugs and biological tissues.
3. Surgical auxiliary materials: In surgical procedures, gel materials are also commonly used for tissue repair and reconstruction, such as hemostasis, wound closure, and soft tissue repair. Biocompatibility studies help evaluate the safety and effectiveness of related products during surgery.
JJRLAB can conduct animal experiments (biocompatibility testing) related to mice, rats, guinea pigs, gerbils, rabbits, dogs, pigs, and monkeys, and can construct more than 200 types of animal disease models.
Email:hello@jjrlab.com
Write your message here and send it to us
 What Certifications Are Required to Sell Air Fryer
What Certifications Are Required to Sell Air Fryer
 EU Toy Safety Standard EN 71-1:2026
EU Toy Safety Standard EN 71-1:2026
 Introduction to EU Toy Standards EN 71‑1 to EN 71‑
Introduction to EU Toy Standards EN 71‑1 to EN 71‑
 Amazon Canada Site / SOR Children's Products Compl
Amazon Canada Site / SOR Children's Products Compl
 How Do You Get a CE Mark
How Do You Get a CE Mark
 IEC 60529 IP Rating Ingress Protection Standard
IEC 60529 IP Rating Ingress Protection Standard
 IEC 60601-1 Medical Electrical Equipment Basic Saf
IEC 60601-1 Medical Electrical Equipment Basic Saf
 European Authorized Representative Medical Devices
European Authorized Representative Medical Devices
Leave us a message
24-hour online customer service at any time to respond, so that you worry!




